Selling unit: per 100ml
Product Name
- Common name: Albumin (ALB) determination kit (bromocresol green method)
- English name: ALB Reagent Kit (BCG Colorimetric Method)
Reagent Ratio
Single reagent
Intended Use
This kit is used to determine the amount of ALB in human serum.
ALB is an important binding and transport protein for a variety of substances in plasma and is a major component in the maintenance of plasma osmolality. Increases are seen in: dehydration and shock due to severe diarrhea and vomiting, as well as dehydration caused by insufficient water intake or extensive burns, acute hemorrhage and Addison’s disease. Decrease is seen in: excessive protein loss, such as nephrotic syndrome, severe bleeding and extensive burns, as well as pleural and abdominal effusions; wasting diseases, such as malignant tumors and hyperthyroidism; decreased albumin synthesis, such as chronic liver diseases, especially in the deterioration of hepatic steatosis, acute and subacute severe hepatitis; insufficient protein intake, such as chronic gastrointestinal disorders with chronic starvation or poor digestion and absorption.
Test Principle
Bromocresol green (BCG) forms an oxal green complex with albumin in serum at pH 4.2 and in the presence of the nonionic detergent Brij-35, and this complex has an absorption peak at 604 (540~610) nm. In a certain content range, the color of this complex is proportional to the content of ALB.
Main Components
Reagent components included in the product.
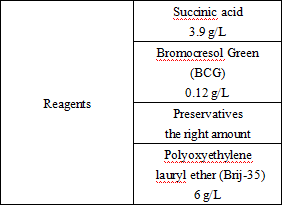
*Components are not interchangeable in kits with different lot numbers.
*Reagent components not included in the product, but necessary for the test: outsourced normal and abnormal QCs and calibrators.
Storage Conditions And Expiration Date
The kit is stored at 2-8°C away from light and is valid for one year.
Reagents that have been opened are taken care not to be contaminated and the reagents are stabilized in the instrument compartment (2-8°C) for one month.
The reagents must not be frozen.
Applicable Instruments
Hitachi 7180/7600; Olympus AU680/2700; Toshiba TBA120; Myriad BS2000M/480; Siemens ADVIA1800/2400 automatic biochemical analyzer.
Sample Request
Serum, which should be separated promptly after venous blood collection.
The albumin in the samples is stable for 1 week at room temperature (18-25°C), 1 month under refrigerated (2-8°C) conditions, and longer under frozen (-20°C) conditions.
Test Method
- Reagent preparation: liquid reagents are ready to use out of the bottle.
- Test conditions: ( The following parameters are set in accordance with Toshiba TBA120FR, can be requested according to different testing instruments for different on-board parameters)
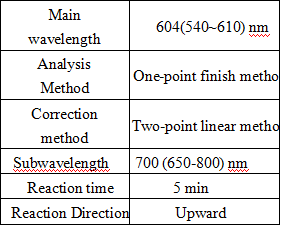
Operation steps.

*Reagents and sample volumes can be increased or decreased in proportion to the requirements of different biochemical analyzers.
- Calibration procedure.
Randox Laboratories Ltd UK is recommended to use the calibration serum produced by Randox Laboratories Ltd. The Randox calibration serum of choice is Bromocresol Green.
- QC control procedures.
It is recommended to use quality control sera produced by Randox Laboratories Ltd UK, Landau Laboratory Diagnostics Ltd. It is recommended that each laboratory establish its own QC system and select appropriate QCs for quality control. The QC values should be within the specified range. If outside the specified range, it is necessary to take appropriate measures or contact the manufacturer.
- Calculation.
ALB concentration (g/L) = (A样品 -A空白 ) × C标准 / (A标准 -A空白 )
Reference Interval
40~55 g/L
The reference interval is derived from the National Clinical Laboratory Practice, 4th edition, and is validated by clinical trials of 150 blood samples from a selected group of normal adults. Each laboratory should establish its own normal reference range based on the region and the population.
Interpretation Of Test Results
The serum ALB measurement is only one of the indicators used by clinicians to diagnose patients. Clinicians must also make a comprehensive judgment based on the patient’s physical symptoms, medical history, and other diagnostic items and diagnostic tools.
Limitations Of The Test Method
Ascorbic acid ≤ 30 mg/dl, bilirubin ≤ 40 mg/dL, hemoglobin ≤ 400 mg/dL and celiac disease ≤ 500 mg/dL did not interfere with the determination.
Product Performance Index
Appearance: The appearance of the kit should be neat and tidy, with clear identification of words and symbols, and the reagent bottle intact; the reagent is a yellow-brown clarified liquid, without precipitation, suspension and flocculent.
Absorbance of reagent blank: wavelength 604 nm, optical diameter 1.0 cm, temperature 37°C, A0 ≤ 0.30.
Analytical sensitivity: the absorbance change value ΔA≥ 0.02 when the kit tests 2 g/L of the analyte.
Linearity interval: For testing serum samples, the linear correlation coefficient|r| should be not less than 0.990 in the interval of 1.0-60.0 g/L. In the interval of [1.0-20.0] g/L, the absolute deviation of linearity should not exceed 4.0 g/L; in the interval of (20.0-60.0] g/L, the relative deviation of linearity should not exceed ±10%.
Precision: repeatability CV≤ 2.0%; relative extreme difference between batches≤ 5.0%.
Accuracy: Relative deviation ≤ 6.0%.
Caution
- This product is for in vitro diagnosis only.
- Avoid contamination when using the reagent, the container used must be clean, and please take necessary precautions, do not swallow, and avoid contact with skin and mucous membrane.
- Do not mix R1 and R2 into a single reagent.
- When changing the reagent lot number, please recalibrate.
- There may be differences in the use of reagents from different manufacturers for the same sample.
- All sample cassette reaction waste should be treated as an infectious source and the operator needs to take the necessary protective measures.
- The reagent contains the preservative sodium azide (toxic), so please rinse thoroughly with water immediately if you accidentally get it in your eyes or mouth or on your skin, and seek medical attention if necessary. Sodium azide can react strongly with copper, lead and other metals to form azide metal, so please dilute the waste solution and flush the drainage pipe when disposing to avoid residue in the drainage pipe.
- test tubes and other instruments that have come into contact with the test sample should be disposed of in accordance with medical waste disposal methods.
- High lipid serum can cause false positive elevation of the results. In this case, a sample blank tube, i.e. 300 μl of 0.9% saline with 3 μl of sample mixed, should be used to read the absorbance of the sample blank tube at 600 nm relative to the 0.9% saline, and the actual sample absorbance should be simply subtracted from the absorbance of the sample blank tube.
- When the concentration of albumin in the sample exceeds 60 g/L, the sample should be diluted with 0.9% saline and then measured, and the result measured multiplied by the dilution factor.
Reference
- Tietz, N.W., Textbook of Clinical Chemistry 2nd ed.
- Doumas, B.T., Watson, W.A. and Biggs, H.G., Clin. Chem. Acta 31:87-96, (1975)
- Webster, D., Clin. Chim. Acta, 53, (1974), 109-115




























