Selling unit: per 100ml
Product Name
Immunoglobulin E assay kit (immunoturbidimetric method)
Package Specification
1) R : 1×30ml R12 : 1×15ml 2) R1 : 2×40ml R2 : 2×20ml
3) R : 2×40ml R12 : 1×40ml 4) R1 : 2×50ml R2 : 1×50ml
5) R : 3×50ml R12 : 3×25ml 6) R1 : 2×90ml R2 : 2×45ml
7) R : 4×60ml R12 : 2×40ml 8) R1 : 5×60ml R2 : 2×50ml
9) R : 2×60ml R12 : 2×20ml 10) R1 : 2×90ml R2 : 2×30ml
11) R1 : 4×40ml R2 : 1×40ml 12) R1 : 2×60ml R2 : 2×15ml
Intended Use
For the in vitro quantitative determination of immunoglobulin E (IgE) levels in human serum (plasma). Increased levels are commonly seen in: allergy-induced rhinitis, rubella, asthma, eczema symptoms; pathological IgE levels can be seen in parasitic diseases and immunocompromised conditions such as acquired T-cell deficiency or wiskott-Aldrich syndrome ; rheumatoid arthritis, systemic lupus erythematosus are also elevated
Test Principle
Immunoglobulin E in the sample combines with specific E antibodies in the reagent to form an insoluble immune complex, causing turbidity in the reaction solution, and the change in turbidity is positively correlated with the amount of E in the sample.
IgE antigen +IgE antibody Antigen-antibody complexes
Main Ingredients
R1 : glycine buffer 160 mmol/L; sodium chloride, 94 mmol/L; bovine serum albumin, 3 g/L.
R2 : glycine buffer 160 mmol/L; IgE antibody-coated latex pellet, 0.5 g/L; sodium chloride, 94 mmol/L
Storage Conditions And Expiration Date
It is valid for 12 months in 2℃~8℃ environment.
Applicable Instruments
Biochemical Analyzer
Sample Request
Serum or (heparin or EDTA) anticoagulated plasma, stable for 7d at 2-8°C; 6m at -20°C; 7d at room temperature.
Test Method
Use multi-point non-linear calibration, such as Logit-Log4P, Cubic, Spline and other modes to correct and save the standard curve for calculating the results
1、Basic parameters
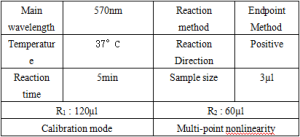
2、Operating steps.
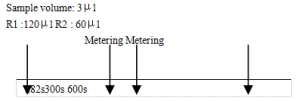
The samples were mixed with R1 and reacted for 282 s. The absorbance A1 was measured, and at 300 s R2 was added, mixed and reacted again for 300 s. The absorbance A2 was measured and the change in absorbance (△A) was calculated.
Calculation Of Test Results
According to the standard curve saved after calibration, the concentration corresponding to the change in absorbance of the measured sample on this curve is consulted, which is the measured concentration.
Reference Range
(0~358) IU/mL.
It is recommended that each laboratory establish its own normal reference range.
Interpretation Of Test Results
Professionals are responsible for the review of test results, the analysis of test results, affected by age, gender, diet, geography, test results such as the appearance of clinical inconsistencies or even contradictions, should be analyzed to find the cause.
Limitations of the test method.
Hemoglobin not more than 5.0g/L and bilirubin not more than 600μmol/L did not interfere with the measurement results.
Product Performance Index
1 Reagent blank absorbance not more than 1.0
2 Accuracy The relative deviation is not more than 15%.
3 Linear range
1) Immunoglobulin E content in the interval 25,1000 IU/ml and the correlation coefficient of linear regression should be not less than 0.990.
(2) Immunoglobulin E content in the interval of 25,75) IU/ml, the absolute deviation ≤ 11.2 IU/ml; in the interval of 75, 1000 IU/ml, the relative deviation ≤ ± 15%.
4 Precision Repeatability: coefficient of variation (CV) not greater than 10%.
Inter-batch difference: Relative polar difference (R) not more than 10%
5 Analytical sensitivity The difference in absorbance of the reactants is not less than 0.01 when the sample concentration is 400 IU/ml.
Caution
1 This product is only used for in vitro diagnosis, and its indicators are used as clinical auxiliary diagnosis.
2 If the specimen concentration exceeds 1000 IU/ml, please dilute it with saline and multiply the result by the dilution times.
3 If the results of the patient’s sample assay during the medication period differ significantly from the clinical diagnosis, it is recommended to discontinue the medication and resample for testing.
4 The sample volume of reagents can be adjusted proportionally according to the different instruments.
Reference
Guo Jianhui. Determination of total serum IgE by latex-enhanced immunoturbidimetric assayJ. Medical Laboratory and Clinical. 2008(05):9-10
Production license number.
Product registration number.
Production and after-sales service units.
Production and registered address.




























