Selling unit: per 100ml
Product Name
- Common name: Immunoglobulin M (IgM) Assay Kit(immunoturbidimetric method)
- English name: IgM Reagent Kit (Immunoturbidimetric Method)
Reagent Ratio
3:1, 4:1, 5:1, common ratio 3:1, other ratios need to be customized
Intended Use
This reagent is used to determine the content of IgM in human serum and plasma.
IgM is one of the earliest immunoglobulins and is the first immunoglobulin synthesized after initial exposure to an antigen. In adult serum, it accounts for 5% of total immunoglobulins. decreased IgM concentrations occur in primary and secondary immunodeficiency syndromes. decreased IgM values are commonly seen in protein-losing intestinal diseases and burns. Severe infections and autoimmune diseases can lead to increased IgM concentrations. A variety of myeloma, macroglobulinemia, bacterial and parasitic infections, liver disease, rheumatoid arthritis, and cholecystic fibrosis can increase IgM concentrations. Elevated serum levels of IgM in neonates imply the development of clinical infections.
Test Principle
IgM and its corresponding antibody meet in the liquid phase to form an insoluble immune complex, resulting in turbidity in the reaction solution, and the turbidity level reflects the IgM content in the serum sample.
Main Components
Reagent components included in the product.
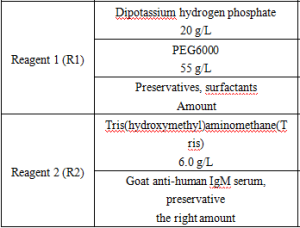
*Components are not interchangeable in kits with different lot numbers.
*Reagent components not included in the product, but necessary for the test: outsourced normal and abnormal QCs and calibrators.
Storage Conditions And Expiration Date
The kit is stored at 2-8°C away from light and is valid for one year.
Reagents that have been opened are careful not to be contaminated, and reagents are stable in the instrument compartment (2-8°C) for one month.
The reagents must not be frozen.
Applicable Instruments
Hitachi 7180/7600; Olympus AU680/2700; Toshiba TBA120; Myriad BS2000M/480; Siemens ADVIA 1800/2400 series automatic biochemical analyzers.
Sample Request
Plasma anticoagulated with serum or heparin should be separated within 2 hours after blood collection and stored at room temperature (15-25°C) for 2 months, 2-8°C for 4 months, and -20°C for 6 months.
Test Method
- Reagent preparation: liquid reagents are ready to use out of the bottle.
- test conditions: (different parameters on the machine can be requested according to different testing instruments)

Operation steps.
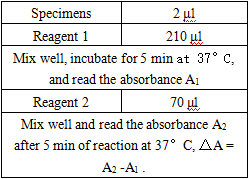
Reagents and sample volumes can be increased or decreased in proportion to the requirements of different biochemical analyzers.
- Calibration procedure.
Landau calibrators are recommended.
- QC control procedures.
It is recommended that each laboratory establish its own quality control system and select appropriate quality control products for quality control. The measured values of QC products should be within the specified range. If outside the specified range, it is necessary to take appropriate measures or contact the manufacturer.
- Calculation.
5 points are calibrated and the corresponding values are entered. With △A as the vertical coordinate and concentration as the horizontal coordinate, the standard curve is plotted, and this standard curve is corrected by the non-linear method Spline, Logit-Log4P and other modes, and this standard curve is saved and used to calculate the results.
Positive Judgment Value Or Reference Interval
0.4~2.3 g/L
It is recommended that each laboratory establish its own reference range of normal values.
Interpretation Of Test Results
Bilirubin ≤ 40 mg/dL, hemoglobin ≤ 400 mg/dL and celiac ≤ 500 mg/dL did not interfere with the determination.
Limitations Of The Test Method
The determination of IgM in human serum or plasma is only one of the indicators used by the clinician to make a diagnosis of the patient. The clinician must also make a comprehensive judgment based on the patient’s physical symptoms, medical history, and other diagnostic items and tools.
Product Performance Index
Absorbance of reagent blank: wavelength 340 nm, optical diameter 1.0 cm, temperature 37°C, A0 ≤ 0. 15.
Analytical sensitivity: the absorbance change value ΔA≥0.1 when the kit tests 2 g/L of the analyte.
Linearity range: test serum samples, reagent linearity in the interval of 0.05~5.00 g/L, linear correlation coefficient|r| should not be less than 0.975; in the interval of 0.05~2.00 g/L, the absolute deviation of linearity should not exceed 0.20 g/L; in the interval of (2.00~5.00 g/L, the relative deviation of linearity should not exceed ±10%.
Precision: repeatability CV≤ 10.0%; relative extreme difference between batches≤ 15.0%.
Accuracy: Relative deviation ≤ 10%.
Caution
- This product is for in vitro diagnosis only.
- Avoid contamination when using the reagent, the container used must be clean, and please take necessary precautions, do not swallow, and avoid contact with skin and mucous membrane.
- Please dispose of the measured samples and waste liquids in accordance with the relevant national and local laws and regulations.
- For samples with lipid blood visible to the naked eye and triglyceride content exceeding 250 mg/dL, the results need to be diluted with saline before determination and multiplied by the dilution factor.
- Please dispose of the measured samples and waste liquids in accordance with the relevant national and local laws and regulations.
- When changing the reagent lot number, the calibration should be re-calibrated.
Reference
- Gitlin D, Edelhoch HJ. Immunol. (1991); 66: 76-78.
- Burtis CA, Ashwood ER. Tietz Fund. of Clin. Chem. 5thed. 30-54 and 462-494.
- Guder WG, Narayanan S, Wisser H, Zawta B. List of Analysis; Pre-analytical Variables. Brochure in: Samples: From the Patient to the Laboratory. Darmstadt: GIT Verlag. (1996).
- Consensus values of the Deutsche Gesellschaft fur Laboratoriums-medizin, the Deutsche Gesellschanft fur Klinische Chemie and the Verband der Diagnostica-Industrie. V. (VDGH). DG Klinische Chemie Mitteilungen (1995): 41: 743-748.




























