Selling unit: per 100ml
Product Name
- Common name: Pepsinogen II assay kit (latex-enhanced immunoturbidimetric assay)
- English name: PepsinogenⅡ (PGⅡ) Assay Kit (Immunoturbidimetric Method)
Reagent Ratio
5.4:1
Intended Use
This kit is used for the in vitro quantitative determination of pepsinogen II (PG II) in human serum or plasma.
PGI originates from the main cells of the fundic glands and the cervical mucus cells, while PGII originates from the whole gastric glands (cardia, fundus, sinus pylorus) and the distal duodenal Brunner’s gland, with the prostate and pancreas also producing small amounts of PGII. Most of the synthesized PG enters the gastric lumen and is activated into pepsin by the action of acidic gastric juice, while only a small amount (about 1%) of PG enters the blood circulation through the gastric mucosal capillaries. Progressive decrease in PGI/II ratio was associated with progression of gastric mucosal atrophy.
Test Principle
The PGII in the sample and the antibody adsorbed by the emulsion particles cause an antigen-antibody reaction, forming an immune complex. The change in turbidity is measured at 700 nm wavelength and the degree of change is proportional to the PGII content of the sample.
Reagent components included in the product.

*Components are not interchangeable in kits with different lot numbers.
*Reagent components not included in the product, but necessary for the test: outsourced normal and abnormal QCs and calibrators.
Storage Conditions And Expiration Date
The kit is stored at 2-8°C away from light and is valid for one year.
Reagents that have been opened are careful not to be contaminated, and reagents are stable in the instrument compartment (2-8°C) for one month.
The reagents must not be frozen.
Applicable Instruments
Hitachi 7180/7600; Olympus AU680/2700; Toshiba TBA120; Myriad BS2000M/480; Siemens ADVIA 1800/2400 series automatic biochemical analyzers.
Sample Request
Serum or plasma.
Samples should be stored away from light.
Test Method
- Reagent preparation: liquid reagents are ready to use out of the bottle.
- test conditions: (different parameters on the machine can be requested according to different testing instruments)

Operation steps.
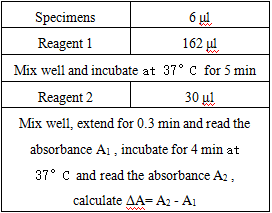
Reagents and sample volumes can be increased or decreased in proportion to the requirements of different biochemical analyzers.
- Calibration procedure.
The use of matching calibrators is recommended.
- QC control procedures.
It is recommended that each laboratory establish its own quality control system and select appropriate quality control products for quality control. The measured values of QC products should be within the specified range. If outside the specified range, it is necessary to take appropriate measures or contact the manufacturer.
- Calculation.
The standard curve is made by the concentration of the standard to the corresponding ΔA. The concentration of PGⅠ in the sample is read out from the standard curve by the ΔA of the sample.
Positive Judgment Value Or Reference Interval
Based on serum PG I and PG I/ I I ratio, the progression of gastric mucosal atrophy was judged as follows.
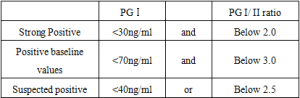
It is recommended that each laboratory establish its own reference range of normal values.
Interpretation Of Test Results
Hemoglobin ≤ 500mg/dL, ascorbic acid ≤ 30mg/dL, RF ≤ 520IU/ml, formazine turbidity ≤ 2100, jaundice ≤ 50mg/mL will not interfere with this test.
Limitations Of The Test Method
The determination of PGⅠ in human serum or plasma is only one of the indicators used by clinicians to diagnose patients. Clinicians must also make a comprehensive judgment based on the patient’s physical symptoms, medical history, as well as other diagnostic items and diagnostic tools.
Product Performance Index
Absorbance of reagent blank: wavelength 700 nm, optical diameter 1.0 cm, temperature 37°C, A0 ≤ 1.0.
Analytical sensitivity: The kit tests 14.8 ng/mL of the analyte with absorbance change value ΔA ≥ 0.015.
Linearity range: test serum samples, reagent linearity in the interval of 2.0 ng/mL ~ 70 ng/mL, linear correlation coefficient|r| should not be less than 0.990; in the interval of 2.0 ~ 10 ng/mL, the absolute deviation of linearity should not exceed 1 ng/mL; in the interval of (10 ~ 70 ng/mL, the relative deviation of linearity should not exceed ±10%.
Precision: repeatability CV ≤ 10%; relative extreme difference between batches ≤ 10%.
Accuracy: Relative deviation ≤ 10%.
Caution
- This product is for in vitro diagnosis only.
- Avoid contamination when using the reagent, the container used must be clean, and please take necessary precautions, do not swallow, and avoid contact with skin and mucous membrane.
- Please dispose of the measured samples and waste liquids in accordance with the relevant national and local laws and regulations.
- When changing the reagent lot number, the calibration should be re-calibrated.
- The reagent may contain infectious microorganisms such as HB virus, and there may be a risk of infection, so care should be taken when using it.
- Do not mix kits of different lot numbers and reagents left after use.
Reference
- Sugawara T.: Medicine and Pharmacy, 43(3), 587-595, (2000)
- Kawamura, T.: Journal of the Japanese Society for Diagnostic Testing of the Digestive Group, 38(5). 592-598 (2000)




























