Selling unit: per 100ml
Product Name
- Common name: Angiotensin-converting enzyme (ACE) assay kit (continuous monitoring method)
- English name: ACE Reagent Kit (Continuous Monitoring Method)
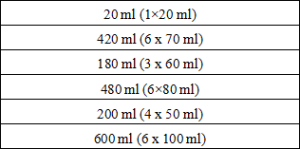
Package Specification
Intended Use
This kit is used to determine the content of ACE in human serum and plasma.
Serum ACE is mainly derived from pulmonary capillary endothelial cells, which can change significantly during acute lung injury, and the change in serum ACE can be used as an important indicator of the extent of lung damage caused by various factors.
Test Principle
ACE
FAPGG ───→ FAP + GG
FAPGG has a maximum absorption peak at wavelength 340 nm, and the application of angiotensin-converting enzyme enzymatically cleaves N-(3[2-Furyl]Acryloy)-phe-gly-gly into FAP and GG, which results in a decreasing change in absorbance at 340 nm. The angiotensin-converting enzyme activity can be calculated by measuring the rate of decrease in absorbance of FAPGG at 340 nm.
Main Components
Reagent components included in the product.

*Components are not interchangeable in kits with different lot numbers.
*Reagent components not included in the product, but necessary for the test: outsourced normal and abnormal QCs and calibrators.
Storage Conditions And Expiration Date
The kit is stored at 2-8°C away from light and is valid for one year.
Reagents that have been opened are careful not to be contaminated, and reagents are stable in the instrument compartment (2-8°C) for one month.
The reagents must not be frozen.
Applicable Instruments
Hitachi 7180/7600; Olympus AU680/2700; Toshiba TBA120; Myriad BS2000M/480; Siemens ADVIA 1800/2400 series automatic biochemical analyzers.
Sample Request
- serum or plasma, blood should be separated promptly after collection to avoid hemolysis; plasma can only be anticoagulated with heparin, not EDTA.
- Specimens stored in ACE at 2-8°C can be stable for 7 days and -20°C for several months.
Test Method
- Reagent preparation:Liquid reagents are ready to use out of the bottle.
- test conditions: (different parameters on the machine can be requested according to different testing instruments)
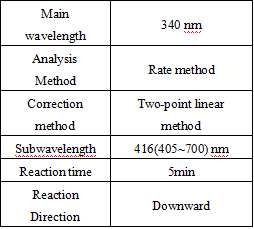
Operation steps.

Reagents and sample volumes can be increased or decreased in proportion to the requirements of different biochemical analyzers.
- Calibration procedure.
The use of matching calibrators is recommended.
- QC control procedures.
It is recommended that each laboratory establish its own quality control system and select appropriate quality control products for quality control. The measured values of QC products should be within the specified range. If outside the specified range, it is necessary to take appropriate measures or contact the manufacturer.
- Calculation.
Sample concentration = (ΔA样品 /min-ΔA空白 /min) × C标准 / (ΔA标准 /min-ΔA空白 /min)
Positive Judgment Value Or Reference Interval
8 to 68 U/L
It is recommended that each laboratory establish its own reference range of normal values.
Interpretation Of Test Results
Bilirubin ≤ 30 mg/dL, bilirubin ≤ 40 mg/dL, hemoglobin ≤ 500 mg/dL and celiac ≤ 500 mg/dL will not interfere with this test.
Limitations Of The Test Method
The determination of ACE in human serum or plasma is only one of the indicators used by the clinician to make a diagnosis of the patient. The clinician must also make a comprehensive judgment based on the patient’s physical symptoms, medical history, and other diagnostic items and diagnostic tools.
Product Performance Index
Absorbance of reagent blank: wavelength 340 nm, optical diameter 1.0 cm, temperature 37°C, A0 ≥ 1.0.
Absorbance change rate of reagent blank: △A/min≤ 0.001 at wavelength 340 nm and optical diameter 1.0 cm.
Analytical sensitivity: The kit tests 50 U/L of the test substance with absorbance change rate ΔA/min ≥ 0.005.
Linearity range: test serum samples, reagent linearity in the interval of 10~150 U/L, linear correlation coefficient|r| should not be less than 0.990; in the interval of [10~70] U/L, the absolute deviation of linearity should not exceed 7 U/L; in the interval of (70~100] U/L, the relative deviation of linearity should not exceed ±10%.
Precision: repeatability CV≤ 6.0%; relative extreme difference between batches≤ 10.0%.
Accuracy: Relative deviation ≤ 10%.
Caution
- This product is for in vitro diagnosis only.
- Avoid contamination when using the reagent, the container used must be clean, and please take necessary precautions, do not swallow, and avoid contact with skin and mucous membrane.
- Please dispose of the measured samples and waste liquids in accordance with the relevant national and local laws and regulations.
- When changing the reagent lot number, the calibration should be re-calibrated.
- Do not mix kits of different lot numbers and reagents left after use.
- Hemolysis interferes with the determination, and hemolysis should be avoided as much as possible during the operation.
- Bilirubin has a significant inhibitory effect on ACE, and serum with severe jaundice can cause significantly lower assay results.
Reference
- Fabiny DL:et al: Clin Chem (1971),17:696
- Vasiliades J:et al:Clin Chem (1976),22:1664




























